RESEARCH
Near InfraRed Spectroscopy (NIR)
- From Anharmonicity Research to Molecular Imaging
Our group has made the basic research about the intermolecular interaction with the analysis of overtone and combination tone spectra in a near-infrared region (NIR, wavelength: 700–2500 nm). The overtone and combination tone are caused by an anharmonic term of a molecular vibrational potential. Then we have investigated the intermolecular interactions in a mixed solution by analyzing the changes of frequency, bandwidth, and transition intensity from the ones of isolated molecule.
We also have developed some new NIR instruments. The development of an online analytical instrument for polymer enabled us to analyze the property of the polymer and copolymer with high efficiency and accuracy in real time. In addition, we have collaborated with companies to develop NIR imaging systems. With these systems, we perform the evaluation of tablet quality and the monitoring of the crystallization process for polymers by using chemical imaging.
Now we start the application research for bioimaging. We want to further improve the analytical and imaging techniques by developing our own analytical techniques for spectrum.

Basic Research (Overtone, Combination Tone, and Anharmonicity)
Study on anharmonicity of vibrational potential and mixed solution
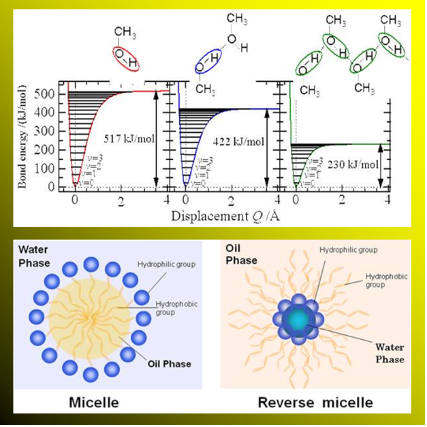
Bands observed in near-infrared region are attributed to overtone and combination tone of normal vibration of a molecule. Because the overtone and combination tone are caused by an anharmonic term of a molecular vibrational potential, knowledge in the anharmonic term of the molecular vibrational potential can be obtained by investigating vibrational energy and intensity of the overtone and combination tone. Therefore, knowledge of intermolecular interaction can be acquired by analyzing the changes of frequency, bandwidth, and transition intensity from isolated molecule.
Our group is mainly aimed at assigning higher-order overtone vibration and understanding interaction between solvent and solute molecules in a liquid and a solid, and has studied intermolecular hydrogen binding between low molecules such as alcohol, acetic acid, and amine, and one between halogenated phenol molecules until now.
As shown in the figure, we demonstrated that a biding energy of the molecular vibrational potential of OH stretching vibration in each hydrogen binding state between methanol molecules in carbon tetrachloride mixture decreases with increasing the molecule combined by the hydrogen binding, and varies with distance from harmonic vibrator approximation.
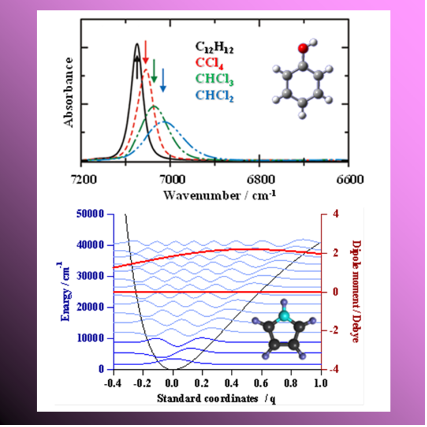
Study of absorption intensity in overtone of molecular vibration
Absorption bands of a main molecule observed with near-infrared region are caused by transition of overtone and combination tone. The overtone transition of a molecular vibration is forbidden transition in harmonic vibrator approximation. However, the molecular vibration of the observed actual spectrum is anharmonic.
Our group has investigated OH stretching vibration of alcohol and phenol, fundamental tone of NH and CO stretching vibrations, frequency of a higher-order overtone (first overtone, second overtone, third overtone etc.), and influence of solvent dependency and hydrogen binding formation on absorption intensity. In addition, we have compared to results of quantum chemical calculation that was considered the anharmonicity of the molecular vibration.
[1] Y. Futami et al., Vib. Spectrosc. 72, pp124–127 (2014).
[2] Y. Chen et al., J. Phys. Chem. A 118, pp2576–2583 (2014).
[3] T. Gonjo et al., J. Phys. Chem. A 115, pp9845–9853 (2011).
[4] Y. Futami et al., J. Phys. Chem. A 115, pp1194–1198 (2011).
[5] Y. Futami et al., Chem. Phys. Lett. 482, pp320–324 (2009).
Development of Equipment, and Imaging
Study of online analysis
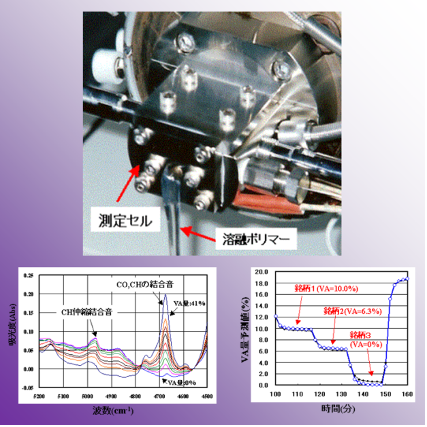
Online analysis is an analytical tool for measuring a sample of a natural state in real-time and then investigating various changes in the sample. In general, since the absorption of near-infrared light is known to be weaker than that of infrared light, an optical fiber can be used for the near-infrared light. The near-infrared light allows a remote sensing in a dangerous area by using the optical fiber. Thus, the online analysis using a spectroscopy in the near-infrared light has attracted much attention in a component analysis in a production process in an industrial field.
On the other hand, to improve products quality in a polymerization process of a polymer and a change management of a brand, achievement of a real-time analytical technique of a polymer has much anticipated. By directly-mounting a cell for the near-infrared spectroscopy in an extrusion device, our group has investigated a property and a component of ethylene-vinylacetate copolymer (EVA) in high efficiency, high accuracy, and real-time.
[1] M. Watari et al., Appl. Spectrosc. 58, pp248–255 (2004).
[2] M. Watari and Y. Ozaki, Appl. Spectrosc. 58, pp1210–1218 (2004).
[3] M. Watari and Y. Ozaki, Appl. Spectrosc. 59, pp600–610 (2005).
[4] M. Watari and Y. Ozaki, Appl. Spectrosc. 59, pp912–919 (2005).
Study of crystalline structure and intermolecular interaction of
a biodegradable polymer using near-infrared spectroscopy
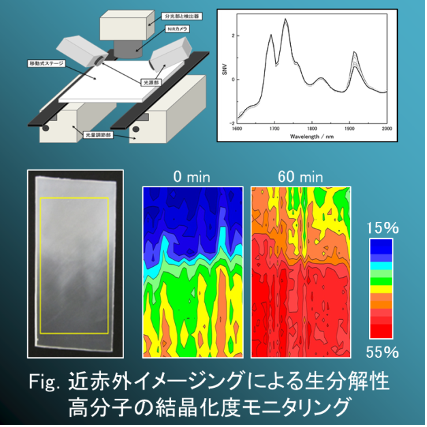
Because of environment issues, biodegradable polymers have been attention recently. Studies of biodegradable polymers are being actively pursued inside and outside Japan. Poly lactic acid (PLA) and poly-(3)-R-hydroxybutyrate (PHB) are well-known biodegradable polymers. Near-infrared (NIR) spectroscopy has been used extensively to predict physical and chemical properties of polymers such as crystallinity, density and concentration.
Since one can apply to a variety of objects which have various physical conditions, shapes and thickness, NIR spectroscopy has often been employed as an efficient method in many practical applications. Therefore, this is why our group decided to research fundamental properties, covering the crystalline structure and thermal behavior of the biodegradable polymers.
Moreover, we not only used the partial least squares (PLS) regression models of crystallintiy had good predicted results on PLA but also used NIR images achieved an obvious contrast of crystallinity around the crystal growth.
[1] D. Ishikawa et al., NIR news 24, pp6–11 (2013).
Study of a quality estimation monitoring of a tablet using near-infrared spectroscopy
In the fine chemicals industry, particularly in the pharmaceutical industry, advanced sensing technologies have recently begun being incorporated into the process line in order to improve safety and quality in accordance with process analytical technology.
For estimating the quality of powders without preparation during drug formulation, near-infrared (NIR) spectroscopy has been considered as the most promising sensing approach. NIR technology, and spectroscopy technology for a spectroscopic analyzer has the required detection mechanism and high sensitivity for powder measurement, as well as a high-speed measuring function for blenders.
Our group has evaluated the characteristics of the developed NIR spectrometer, and confirmed that the measurement of powder samples has high functionality. We also reported the ability to visualize and the quantitative result for target concentration with high accuracy during tablet dissolution by NIR images. This result demonstrates the novel potential in pharmaceutical applications of this new portable NIR imaging instrument.
[1] D. Ishikawa et al., ABC 405, pp9401–9409 (2013).

Bioanalysis
NIR spectroscopy and imaging studies of biological materials
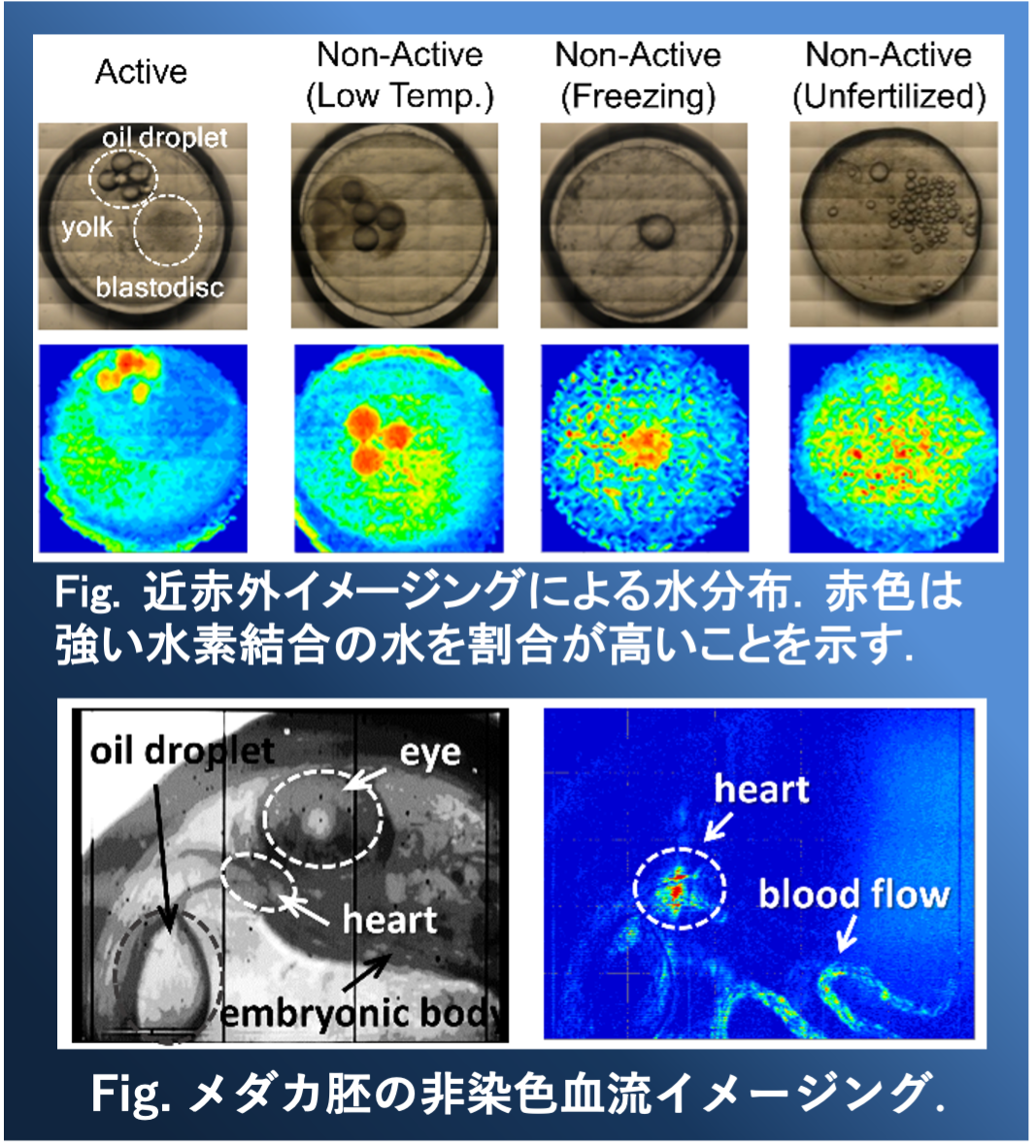
Near-infrared (NIR) spectroscopy can measure samples in non-destructive, non-staining, and non-invasive manners. Since biomolecules such as amino acids, proteins, lipids, and fluorophores exhibit characteristics absorption spectra, molecular structures and intermolecular interactions can be investigated using NIR spectroscopy. Therefore, NIR spectroscopy is applicable as a useful tool for a real-time monitoring of a biological sample.
Our group has tried to apply NIR imaging to biology. In particular, the structural changes of proteins and oxidation of lipids have been investigated in situ. We aim to visualize the biomolecular distribution and the variation of metabolic activity [1-3]. Recently, water structural changes associated with medaka embryogenesis and non-staining blood flow imaging are also studied using NIR imaging [4, 5].
[1] M. Ishigaki et al. Sci. Rep. 6: 20066 (2016)
[2] P. Puangchit et al. Analyst 142: 4765-4772 (2017)
[3] M. Ishigaki et al. J. Biophotonics 11: e201700115 (2018)
[4] M. Ishigaki, M. et al. Anal. Chem. 92: 8133-8141 (2020)
[5] M. Ishigaki, M. et al. Anal. Chem. 90: 5217-5223 (2018)
Spectral Analysis
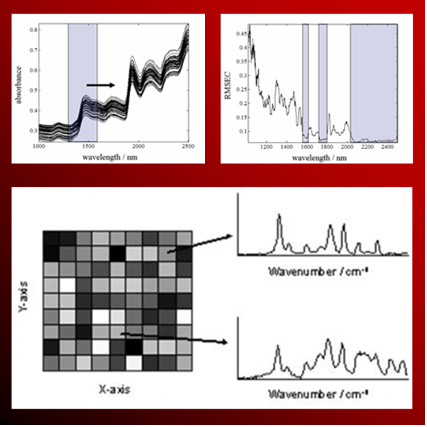
Study of spectral analysis
A variate in chemical data obtained with measurement significantly increased with the progress of electronics and computer. To handle such a multivariate chemical data, “Chemometrics” that was integrated “Chemistry” with “Metrics” was proposed as a discipline for studying that information obtained by applying mathematical and statistical approaches is maximized.
Our group has not only performed spectrum analysis using chemometrics but also developed various one’s own unique chemometric techniques that are better than a traditional technique.
To analyze molecular orientation changing with vibration of a polymeric material from the obtained infrared spectrum, two-dimensional correlation spectroscopy was proposed by I. Noda in Procter and Gamble in 1986.
We have collaborated many researches with I. Noda until now, and have not only analyzed a spectrum but also proposed a new analytical technique of a spectrum. We have also developed a software that everybody is easy to use. Such a software is published on our homepage.
- 1. Near InfraRed Spectroscopy (NIR)
- From Anharmonicity Research to Molecular Imaging -NIR group - 2. Basic Research (Overtone, Combination Tone, and Anharmonicity)
- 2.1 Study on anharmonicity of vibrational potential and mixed solution
- 2.2 Study of absorption intensity in overtone of molecular vibration
- 3. Development of Equipment, and Imaging
- 3.1 Study of online analysis
- 3.2 Study of crystalline structure and intermolecular interaction of
a biodegradable polymer using near-infrared spectroscopy - 3.3 Study of a quality estimation monitoring of a tablet using near-infrared spectroscopy
- 4. Bioanalysis
- 4.1 NIR spectroscopy and imaging studies of biological materials
- 5. Spectral Analysis
- 5.1 Study of spectral analysis
CONTESTS